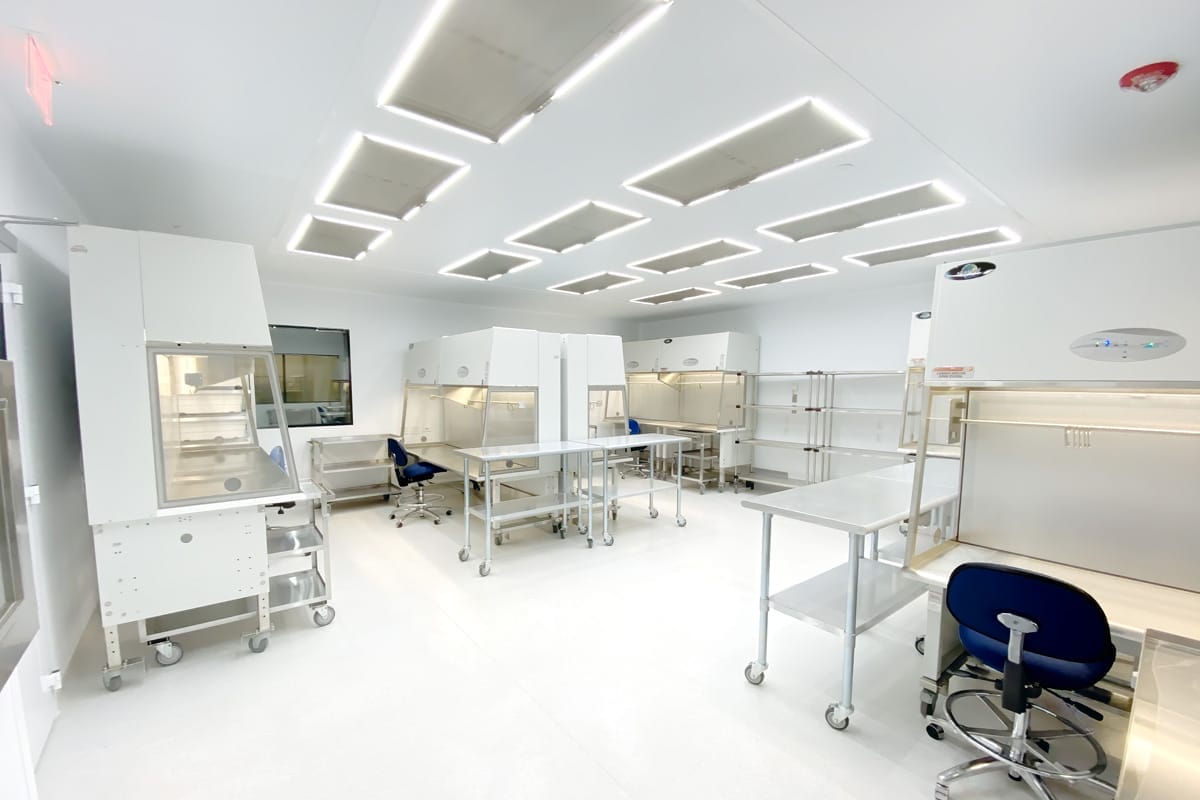
STERILE CLEANROOMS
For Pharmacies, Hospitals and Manufacturers
Aseptic Construction Methods
CE Cleanrooms has unrivaled expertise in the end-to-end design/build process for compliant aseptic cleanrooms for pharmacy and health care applications around the US.
We build facilities for a wide range of manufacturing processes and needs, including medical devices and hazardous drugs. In addition, we have highly specialized niche expertise in providing cleanrooms for IV-compounding pharmacies.
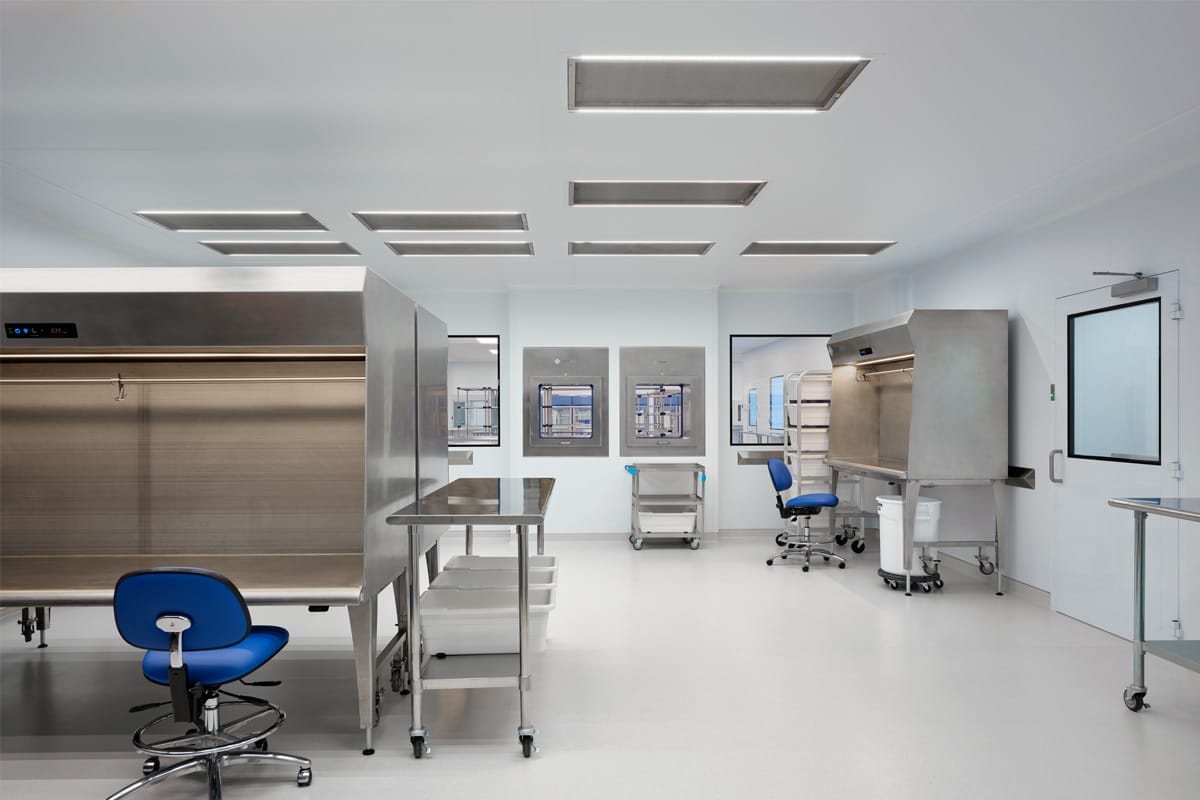
Focused on End-User Efficiency and Productivity
To enhance productivity and efficiency, our architects design the most effective environment for your workflow patterns. Our commitment to value engineering means we optimize every cleanroom to make the best use of your space ensuring it meets the needs of your processes, people and equipment.
Meeting all Regulations and Compliance Requirements
The CE Cleanrooms' team designs and builds your critical environment to meet – and even exceed – all necessary standards and associated nuances to help you avoid costly inspection problems that delay – or even prevent – certification.
Our sterile cleanrooms are built to comply with USP <795, 797/800 & 825> and FDA 503A and 503B standards, although the exact compliance profile for each facility depends on the scale of the operation, whether pharmacists are developing prescriptions customized for a specific patient and whether the drugs are on the NIOSH list.
Once your cleanroom is completed, the CE Cleanrooms' team executes a detailed commissioning checklist to ensure all construction features, including the mechanical systems, airflow, monitoring and contamination control devices, are optimized, balanced and working correctly before going through any certification processes.
We provide referrals to third-party certification companies and, on the rare occasion when something needs to be adjusted, your CE construction team will bring it up to the required standard.

Governing Bodies
Third-party Accreditation for Facilities
Ongoing Certification
Click images to enlarge
Cleanrooms for IV Compounding
The CE Cleanrooms' team has developed unrivaled expertise in designing and constructing multi-location IV-compounding cleanrooms. Our clients include many of the country’s leading retail pharmacies, hospitals and hospital systems as well as regional brands that demand the highest level of construction excellence.
Types of IV-Compounding Cleanrooms
Our facilities are used for the compounding and mixing of customized prescriptions, bulk IV-compound manufacturing and the production of hazardous drugs such as radiopharmaceuticals that contain radioactive isotopes, antineoplastic drugs and other medications on the NIOSH list.

FDA-Validated Pharmaceutical and Therapeutics Manufacturing Cleanrooms
CE Cleanrooms builds highly specialized cleanrooms that provide contamination-free laboratories and manufacturing plants for the safe and effective production of drugs, vaccines and other medical products.
Below are some of the types of pharmaceutical and therapeutics environments we build:
OSD (Oral Solid Dose)
Sterile facilities designed to accommodate the workflow, machinery and personnel needed to manufacture a wide variety of pills and tablets, including everything from controlled substances to nutrients to over-the-counter vitamins.
Blow Fill Seal
Aseptic manufacturing environments designed to meet injection molding process requirements where ultra-clean plastic bottles get filled with sterile drugs or solutions and are then sealed to prevent contamination.
Pharmaceutical Injectable
Germ-free industrial manufacturing plants where precision equipment fills vials with injectable drugs.
APIs
Controlled environment spaces for the chemical synthesis, manufacturing and formulation of active pharmaceutical ingredients (APIs) that are needed for pharmaceutical drug manufacturing.
Biopharmaceuticals
Antiseptic research and development labs and manufacturing environments where biopharmaceuticals such as bone, tissue, vaccines, blood components and other products are grown using biological processes rather than chemical synthesis.
Medical Devices
This is a broad category of cleanrooms with features and regulations that vary depending on the device manufactured and its medical use.
Products include everything from plastics, catheters and surgical tools to hoses, silicone breast implants and items transplanted into the body.